Anti-C4d chimeric antigen receptor regulatory T cells suppressed allograft rejection in ABO-incompatible heart transplantation


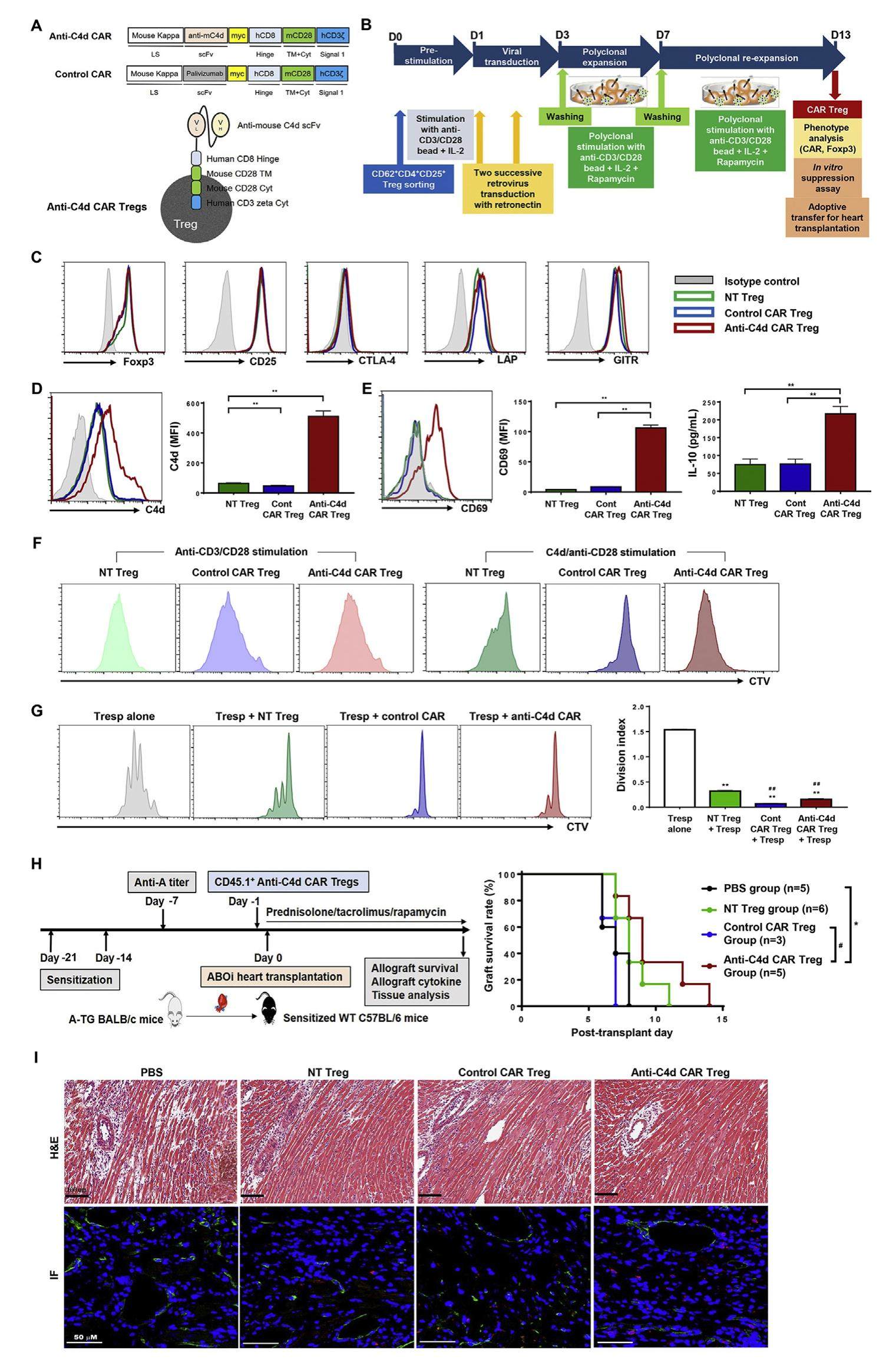
ABO blood group-incompatible (ABOi) transplantation has been developed to overcome the serious problem of donor organ shortage. However, antibody-mediated rejection (ABMR) remains as the main limitation to successful ABOi transplantation. Introduction of desensitization treatment improved the outcomes of ABOi transplantation by suppressing ABMR; however, this strong, nonspecific immunosuppression also increases infectious complications. Recently, chimeric antigen receptor regulatory T cells (CAR Tregs) were developed to improve the antigen specificity, viability, and suppressive activity of Tregs. C4d deposition is a marker of ABMR and is also found in most ABOi allograft tissues. Based on these findings, we developed anti-C4d CAR Tregs to suppress ABMR in ABOi allografts. Anti-C4d CAR Tregs prepared by retroviral transduction of CAR into CD62L+CD4+CD25+ Tregs, expressed forkhead box P3 (Foxp3), CD25, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), latency-associated peptide (LAP), and glucocorticoid-induced tumor necrosis factor receptorrelated protein (GITR) to similar extents as nontransduced Tregs. Anti-C4d CAR Tregs were activated by specific binding to C4d and suppressed in vitro T cell proliferation as well as non-transduced Tregs. Furthermore, adoptive transfer of anti-C4d CAR Tregs significantly prolonged mouse ABOi heart allograft survival (P < 0.05).