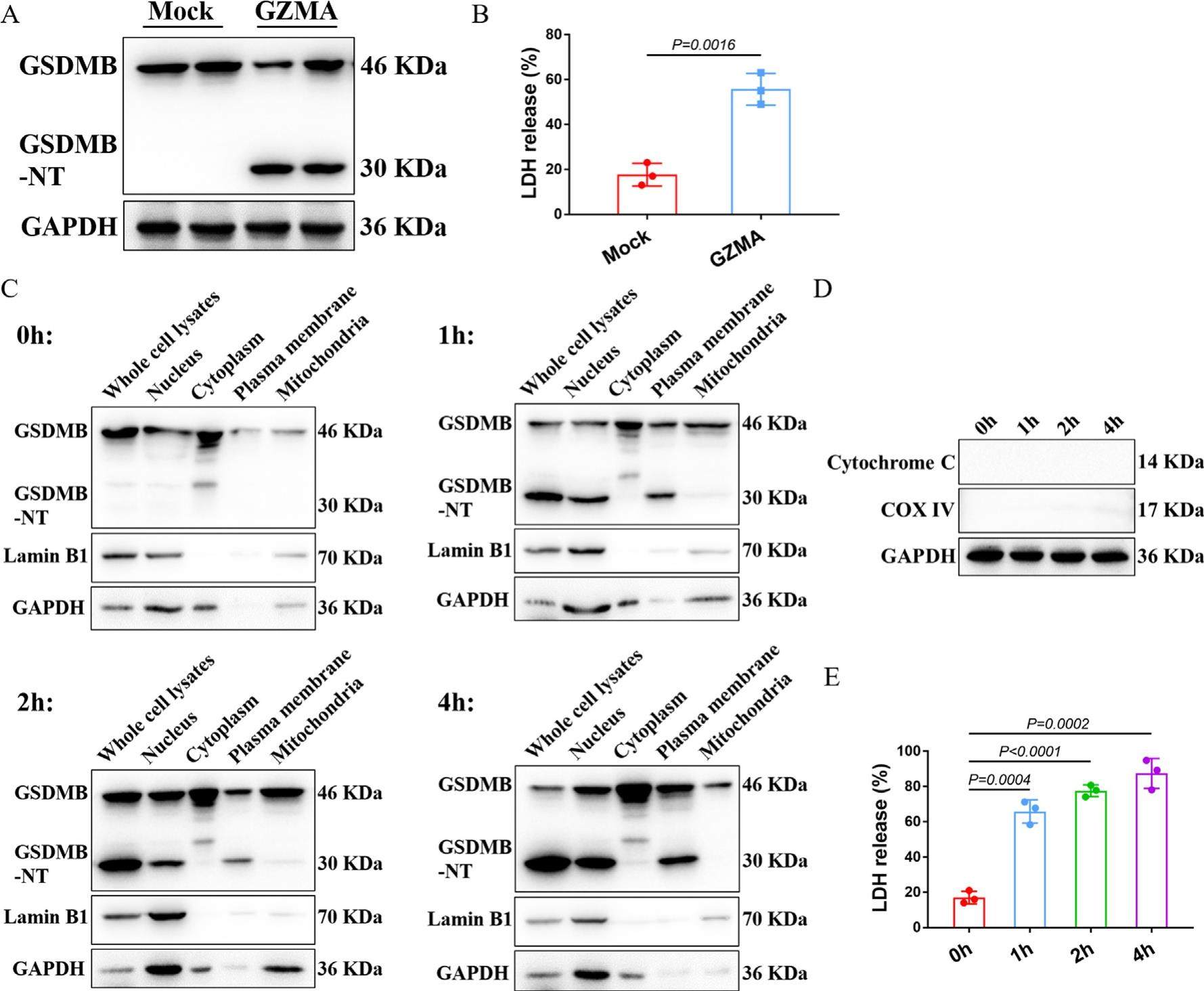
GSDMB N-terminal assembles in plasma membrane to execute pyroptotic cell death


Human genome encodes six paralogous gasdermin genes: GSDMA, GSDMB, GSDMC, GSDMD, GSDME and DFNB59. Proteolytic cleavage of these gasdermin proteins liberates an N-terminal (NT) fragment from autoinhibition, which assembles in membrane to form pores and execute pyroptotic cell death in general. In contrast to other gasdermins, gasdermin B (GSDMB) is the only gasdermin gene that has not been identified in rodents. Zhou et al first shed light on the molecular mechanism by which cytotoxic lymphocyte- derived granzyme A (GZMA) cleaves GSDMB to execute pyroptosis in GSDMB-positive cells, especially in cancer cells. In this issue of Cell, Hansen et al reported a dynamic host pathogen S. flexneri prevents GSDMB-mediated lysis by secreting IpaH7.8, which targets and ubiquitinates GSDMB for 26S proteasome destruction. They showed that GSDMB implements bacteriocidic ability by recognition of the phospholipids on Gram-negative bacterial membranes rather than lysing host cells. Although their experimental design and data are clearly presented and straightforward, there are still some doubts to be clarified.