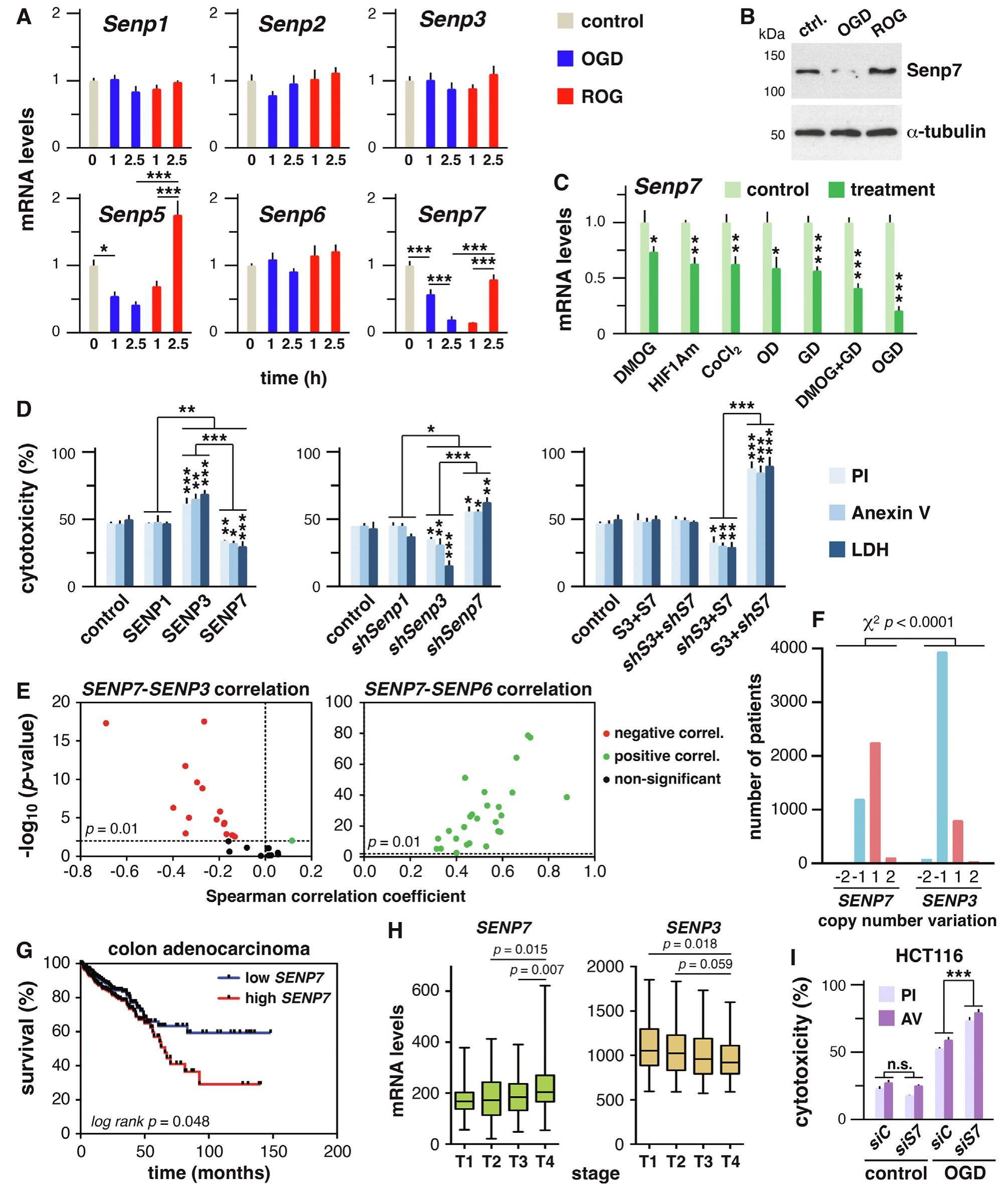
SENP7 overexpression protects cancer cells from oxygen and glucose deprivation and associates with poor prognosis in colon cancer


Constricted oxygen and glucose supplies are common conditions that healthy cells following a stress such an ischemic insult as well as cancer cells within a solid tumor need to overcome in order to survive. These conditions are a serious challenge for cells, which accurately measure the impact to make a life or death decision. In cancer cells, aberrant gene expression and/or protein regulation, frequently linked to appearing mutations, may confer selective advantages to survive under these adverse conditions. In the past years, cumulative evidence indicates that protein modification by covalent attachment of the Small Ubiquitin-like MOdifier (SUMO) plays a role in cell survival after oxygen and glucose deprivation (OGD). Sumoylation involves maturation of SUMO, E1 (SAE1/UBA2)-mediated activation and transfer to the E2 conjugating enzyme (UBC9), to be attached to target proteins, which is frequently facilitated by a E3 ligase, with PIAS as the most investigated proteins. Specific SUMO proteases such as the SENP family members are involved in maturation and recycling SUMO from targets. SUMO is essential in vertebrates, participates in most physiological processes and is associated with cancer. Three functional SUMO molecules have been identified in humans: SUMO1, 2 and 3. SUMO2 and 3, usually referred to as SUMO2/3, are highly homologous and undistinguishable, and are abundant in the cell as unconjugated to rapidly modify proteins in response to OGD. From the initial observation of increased SUMO2/3 conjugation in squirrel brain during hibernation torpor a number of reports have described protection effects of sumoylation in ischemic/reperfusion models, and established better outcomes in cell viability associated with SUMO or UBC9 overexpression and worse outcomes associated with SUMO silencing or SENP1 overexpression. However, sumoylation of heterochromatin protein 1 α (HP1α) has been linked to senescence in cancer cells. To define molecular relevant aspects of SUMO-mediated response to OGD, we have investigated gene expression regulation of the sumoylation pathway components. Notably we found that Senp7 gene and protein are downregulated in OGD-treated cancer cells. Interestingly, SENP7 overexpression under these conditions increased cell survival, while downregulation potentiated OGD-triggered apoptosis. In contrast, SENP3 promoted cell death. These observations are in agreement with prevalent amplification of SENP7 and deletion of SENP3 genomic regions in many cancer types. More importantly, in colon cancer, high expression of SENP7 correlated with both, poor prognosis and higher transformation degree.