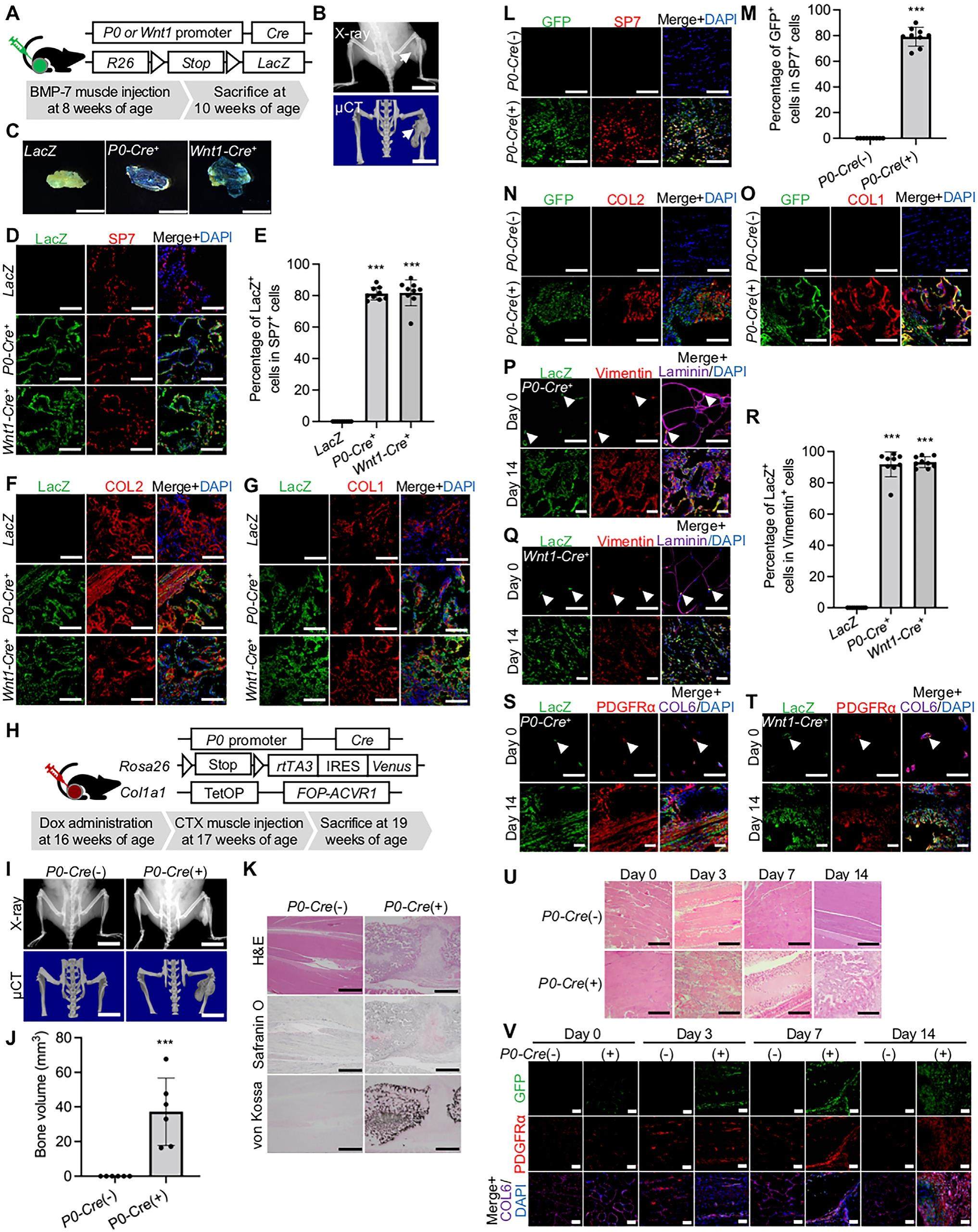
Myelin protein zero (P0) -and Wnt1-Cre marked muscle resident neural crest-derived mesenchymal progenitor cells give rise to heterotopic ossification in mouse models


Heterotopic ossification (HO) describes bone formation at non-skeletal sites and results from traumatic injury, surgery, or genetic disease such as fibrodysplasia ossificans progressiva (FOP). Although it is known that BMP signaling regulates HO, knowledge about the developmental origin of the osteogenic progenitors responsible for the BMP-associated metamorphosis is comparably less. With the use of transgenic mice and labelled neural crest-derived cell, we found myelin protein zero (P0, or MPZ) -and Wnt1-lineage cells give rise to BMP-7 induced adult ectopic cartilage and bone. In addition, the induced expression of ACVR1 (R206H), which is the major mutation found in FOP patients, in P0-lineage cells formed ectopic bone after cardiotoxin-induced muscle injury. We also found that the majority of muscle-resident fibro-adipogenic progenitors (FAPs), essential for muscle homeostasis and responsible for HO in skeletal muscle, are derived from P0- and Wnt1-lineage cells. The data collectively suggest that muscle-resident neural crest-derived progenitor cells account for both nonhereditary and genetic type HO.