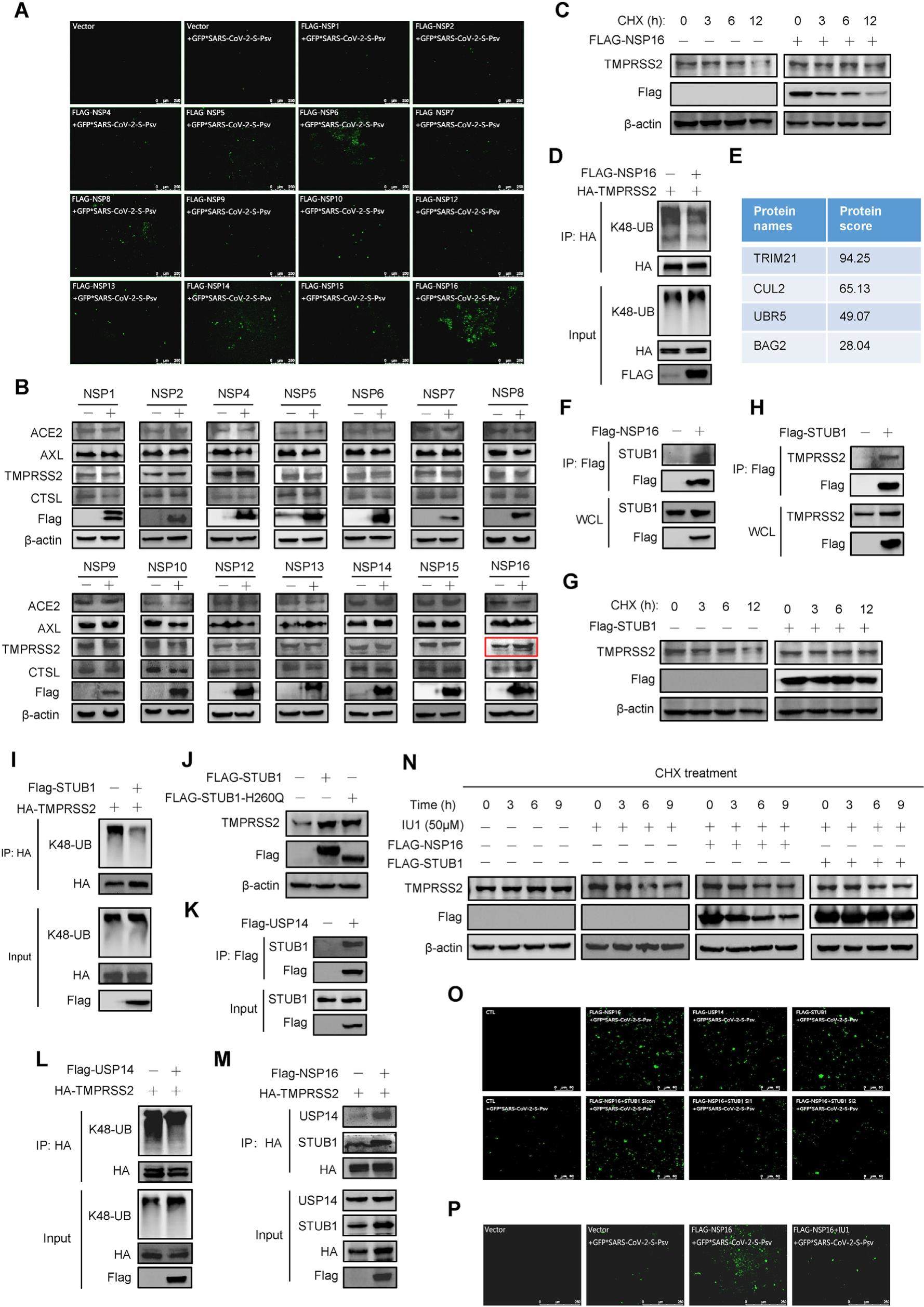
NSP16 promotes the expression of TMPRSS2 to enhance SARS-CoV-2 cell entry


Since the end of 2019, COVID-19 has caused worldwide pandemic. SARS-CoV-2, the culprit of this epidemic, binds to the host receptor Angiotensin converting enzyme 2 (ACE2) using spike (S) protein for cell entry. Recent studies showed that ACE2 expressed at a low level in the main target organs such as lung and bronchial tissues. How SARS-CoV-2 efficiently invades into the respiratory system or other organs with low levels of ACE2 is an urgent problem to be solved. Here, we discovered that NSP16 significantly promoted the invasion of SARS-CoV-2 pseudovirus. NSP16 could promote the formation of a STUB1-USP14 de-ubiquitination complex that regulated the ubiquitination and stability of transmembrane serine protease 2 (TMPRSS2). Inhibiting the function of this complex remarkably reduced the invasion ability of SARS-CoV-2 pseudovirus. Thus, our study demonstrates for the first time that NSPs of SARS-CoV-2 can also participate in viral invasion.