SUV39H1-driven NFATc1 methylation is essential for the c-Cbl-mediated degradation of NFATc1 in an osteoclast lineage


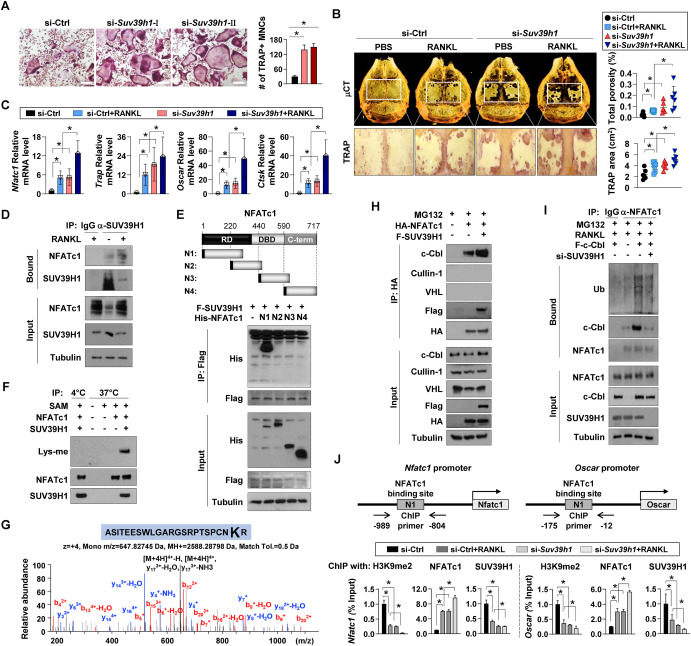
Bone undergoes continuous remodeling by tightly-coordinated actions of bone-resorbing osteoclasts and bone-forming osteoblasts.1 Recent studies document that the dysregulation of histone methylation profiles is associated with the progression of osteoclastogenesis. However, the specific epigenetic modifiers are incompletely understood. In this study, we demonstrate an inhibitory role of a variegation 3–9 homolog 1 (SUV39H1) in osteoclast formation. Public datasets find that mRNA levels of several methyltransferases are gradually decreased during osteoclastogenesis. Among them, a decrease in SUV39H1 mRNA level leads to accelerated osteoclast differentiation with the induction of several osteoclastogenic genes both in vivo and in vitro. In this regard, SUV39H1 directly binds and methylates a nuclear factor of activated T cells 1 (NFATc1) at lysine 267 of the regulatory domain (RD) motif. Of note, SUV39H1 enhances c-Cbl-dependent NFATc1 ubiquitination. Reduced NFATc1 methylation by the knockdown of SUV39H1 significantly decreases c-Cbl-dependent NFATc1 ubiquitination. Finally, SUV39H1 methylates lysine 9 of histone 3 (H3K9) at osteoclastogenic gene promoters, thereby repressing NFATc1 transcriptional activity. Taken together, our findings reveal that SUV39H1 plays both epigenetic and enzymatic roles in its action as an intrinsic suppressor of osteoclastogenesis.