Deletion of heterogeneous nuclear ribonucleoprotein K in satellite cells leads to inhibited skeletal muscle regeneration in mice


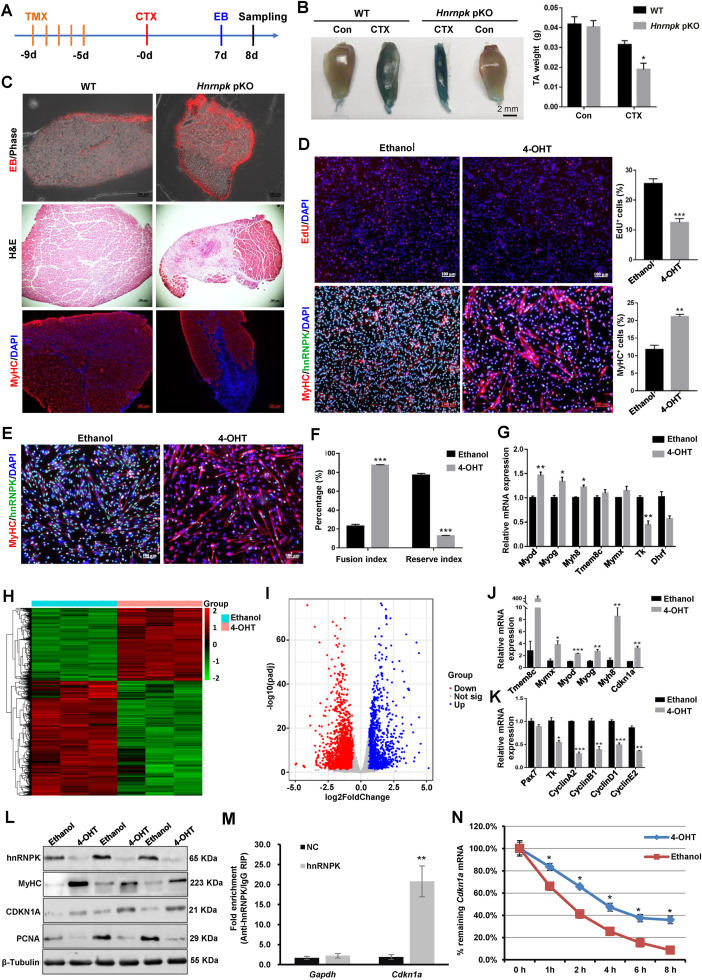
Heterogeneous nuclear ribonucleoprotein K (hnRNPK) is a predominantly nuclear RNA-binding protein that can bind to DNA or RNA through three KH domains and interact with multiple proteins by interactive region. These binding activities enable hnRNPK to link the function in a wide array of diverse cellular processes, such as chromatin remodeling, gene transcription, RNA metabolism, protein translation, DNA repair, and cell signal transduction, thereby playing crucial roles in many biological processes, including development, axonal regeneration, spermatogenesis, cell cycle, apoptosis, differentiation, and carcinogenesis.1 Lack of hnRNPK in C2C12 myoblasts results in the decreased proliferation rate of myoblasts and an inhibitory effect on muscle differentiation. Homozygous Hnrnpk knockout (Hnrnpk−/−) mice are embryonic lethal, suggestive of its decisive role in development and neonatal survival.2 In addition, mutation of HNRNPK in humans causes Au-Kline syndrome which is a rare neurodevelopmental multiple congenital malformation syndrome associated with global developmental delay, characteristic facies, congenital heart defects, skeletal abnormalities, and muscle weakness.3 These findings demonstrate that hnRNPK plays a critical role in skeletal muscle development and myogenesis. However, the molecular mechanism of hnRNPK in skeletal muscle development has not been convincingly demonstrated, especially in animal models. Here, we constructed an hnRNPK-inducible skeletal muscle satellite cell-specific knockout mouse model and found that hnRNPK depletion in mice inhibited muscle regeneration, emphasizing the importance of hnRNPK in myogenesis. Further research demonstrated that hnRNPK may feature in skeletal muscle regeneration via binding Cdnk1a 3′UTR to modulate Cdnk1a mRNA stability. These findings suggested that hnRNPK is required for muscle regeneration and might be a potential novel target for the treatment of muscle disorders.