Complement C1q induces the M2-polarization of tumor-associated macrophages in lung adenocarcinoma


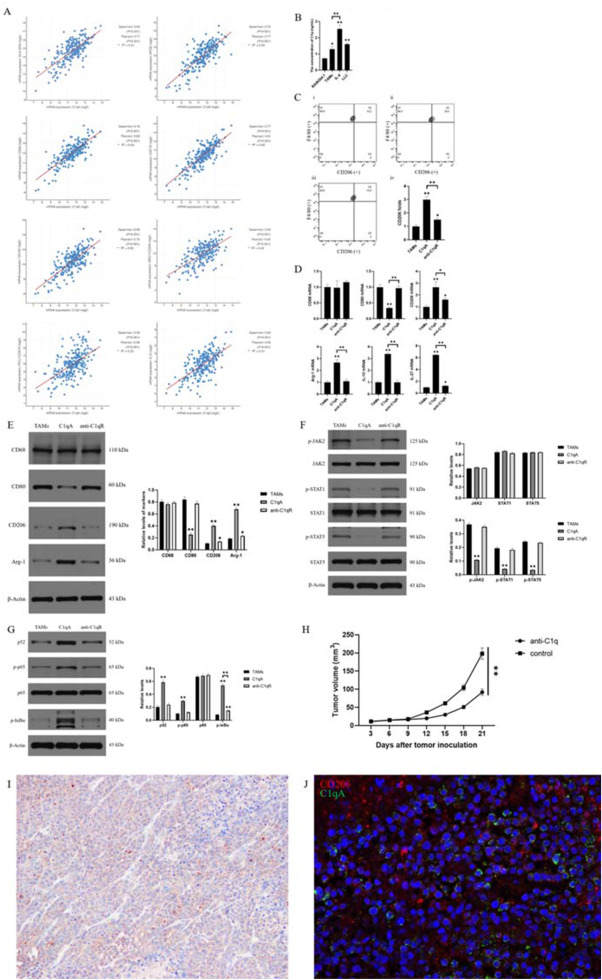
Complement C1q was proved to be able to regulate the polarization of macrophages to the anti-inflammatory phenotype (M2 polarized), acting as an anti-inflammation molecule independent of the classical complement pathway. A high level of C1q expression has been detected in the tumor microenvironment (TME) of diverse tumors, including non-small cell lung cancer, in which C1q could be considered a predictor of poor prognosis.1 Prominently, researchers have found that C1q was positively correlated with the M2 polarization of tumor-associated macrophages (TAMs) in mouse renal clear cell carcinoma and human osteosarcoma. M2-TAMs are significantly associated with immunosuppressive TME and poor prognosis in non-small cell lung cancer. Hence, we investigated the mutual effects between C1q and TAMs in lung adenocarcinoma. The findings of this study could be extracted as below: (i) The expression of C1qA was positively correlated with M2-TAMs in lung adenocarcinoma. (ii) M2-TAMs could express abundant C1q, and C1q in lung adenocarcinoma was mainly from TAMs, especially M2-TAMs. (iii) C1qA could promote the proportion of M2-TAMs. (iv) C1qA led to the reduced phosphorylation of JAK2, STAT1, and STAT5, but augmented the levels of p52, phospho-p65, and phospho-IκBα belonging to NF-κB pathway. (v) C1q promoted the growth of LLC xenograft.