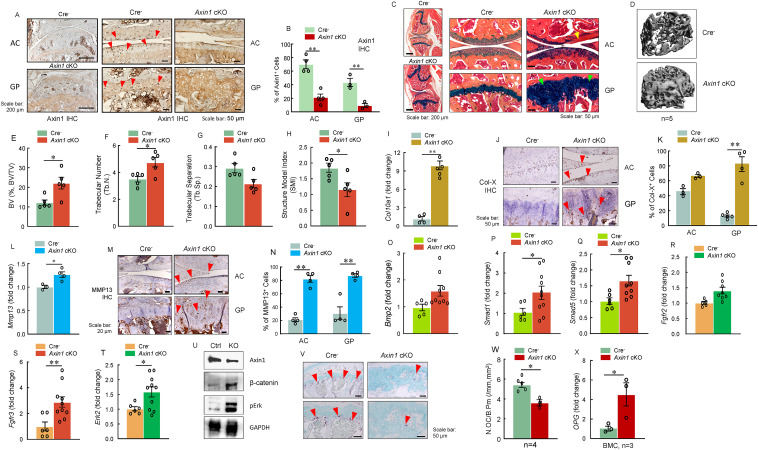
Deletion of Axin1 in aggrecan-expressing cells leads to growth plate cartilage defects in adult mice


In mammals, skeletal bone development begins at hyaline cartilage formation at the early embryonic stage.1 Then osteoblasts from the periosteum, which are distributed surrounding the hyaline cartilage, build up compact bone to form diaphysis and spongy bone known as primary ossification center.1 Next, osteoclasts from the hematopoietic system destroy spongy bone to generate a medullary cavity.1 Along with this continuous establishment of the medullary cavity, after birth, there is a formation of a secondary ossification center occurring at both polar sites (epiphysis) of original hyaline cartilage in a post-axis manner.1, 2, 3, 4 After the formation of the secondary ossification center, the cartilage will be substituted by the spongy bone but leave intact articular cartilage and growth plate (epiphyseal plate) which is comprised of chondrocytes.1 This process is known as endochondral ossification.1 During endochondral ossification, the growth plate exerts a fundamental role in increasing the length of the skeletal bone.1 There are three principal layers of the structure of a growth plate: resting zone, proliferating zone, and hypertrophic zone.2 The cells with mesenchymal stem cell features in the resting zone give rise to clones of proliferating chondrocytes and control the alignment of the proliferating chondrocytes into columns parallel to the long axis of the bone via producing morphogens.2 Proliferating zone is responsible for elongating endochondral bone shape by dividing and arranging clones of chondrocytes into columnar structure.2 Chondrocytes in the hypertrophic zone generated by terminal differentiation of proliferating chondrocytes are contributing to long bone growth by attracting vascular and progenitor cell invasion from adjacent areas.2 Sustained chondrocyte proliferation and hypertrophy lead to calcification and accumulation of bone, thus lengthening the bone during skeletal development and postnatal bone growth.1,2 Unlike humans, there is no termination of proliferation and calcification of chondrocytes in the growth plate in mice. Therefore, it provides us the opportunity to investigate the mechanism of postnatal bone growth.